FAQs on E-Scripts (Electronic Prescriptions)
What has my E script been cancelled?
If your GP has cancelled the prescription, you will need to contact the clinic and let the GP know. The reception staff will let you know if you need to make another appointment with the GP.
Do I need to make an appointment with the doctor for repeat scripts?
Yes. For a repeat prescription, a new appointment with a GP is required.
What should I do if I have not received my first script?
Check your email or phone (depending on if it is sent by email or text) and search for the word ‘prescription’. If you still cannot find it, make a new
appointment with your GP and check with the reception to confirm if the mobile number and / or email provided are accurate.
Why cannot I find my repeat scripts?
If you had the first prescription dispensed and cannot find your repeats, you
need to speak to the pharmacy you attended for the first script to get another token
for repeats. This cannot be done by your GP.
Can I have an e-script and a paper script for the same medication?
No, unfortunately you can only opt for either a paper script or an e-script for the
same medication.
FAQs on Referrals
Do I need to make an appointment with a doctor to renew my referral?
Yes. For a referral renewal a new appointment with a GP is required.
What would the Passcode for opening a Mediref document?
This would be your date of birth DDMM (e.g.: 2nd of March will be 0203)
How would I receive my referral?
Referrals can be delivered by email (MediRef), Secure Messaging, (such as
Healthlink), e-Fax, or may have been printed for in person delivery. We rarely post now.
GPs should in most instances email Specialist Referrals direct to patients via
MediRef and use eFax for Hospital referrals.
I have changed the specialist I am seeing, do I need a new referral?
No. A referral is valid for any other practitioner addressing the problem outlined
in the referral.
A change of details requires another GP appointment.
What if my referral is out of date?
A referral lasts 12 months from the date the Specialist first sees the patient NOT
the date it was written. New referrals however, require a GP appointment.
I have lost referral, what should do I do?
If a referral has been made but the referral has been lost you can still access an initial consultation with a specialist by supplying the name of your GP, the practice address or Medicare provider number of your GP.
However, for future visits to your specialist a replacement referral must be made. You can request a referral from your GP by booking an appointment here – Hobsons Bay | Basywater
How long does a referral last and when do I need a new referral?
If supplied by a GP, the law states that a referral is valid for a single course of treatment for a period of 12 months after the first service, although the GP can indicate an alternative time period (e.g 3, 6, 18 months or indefinite).
Note the referral period begins on the date of the first specialist visit, not on the date the referral was written. This is frequently misunderstood, including by Specialists and their receptionists. If a specialist or their reception staff request a new referral when your GP has supplied you with an indefinite referral for a particular condition, you do NOT need to do so.
Can I use my referral to see someone else?
Yes, you can. It is not a Medicare or legal requirement for a referral to be addressed to a named specialist. If you have a referral that starts with “Dear gastroenterologist” that is acceptable to access the service and also to receive payment from Medicare using that referral.
This is also frequently misunderstood, by Specialists and their receptionists. If you are requested to supply a named referral before you book with or see a specialist know that you do not have to do so.
Here are some links to additional information on referrals:
FAQs on COVID 19 Vaccines
Am I eligible for my 4th Covid vaccine? If not, when will I be?
You should get a fourth dose if you are:
● 50 years or older
● A resident of an aged care or disability care facility
● Aeverely immunocompromised (this will be a fifth dose)
● Aboriginal or Torres Strait Islander and aged 50 years and older
● 16 years or older and with a medical condition that increases the risk of severe
COVID-19 illness (see the table below for expanded groups)
● 16 years or older with disability with significant or complex health needs or
multiple comorbidities which increase risk of poor outcome.
ATAGI has advised people aged 30 to 49 years old can receive a fourth dose if they choose.
Why are multi-dose vials being used to store COVID-19 vaccines?
- Multi-dose vials contain more than one dose of a vaccine in a single glass vial. They usually include 5–20 doses per vial, and each dose is then carefully extracted and given via individual syringes for injection. Use of multi-dose vials is the most efficient way to distribute a new vaccine to the maximum number of people and is being used world-wide for all COVID-19 vaccines.
- Packaging vaccine doses multi-dose vials is safe and is supported by numerous quality controls and good handling practices.
- Multi-dose vials are routinely used in Australia for the tuberculosis (BCG) vaccine and were used for the 2009 pandemic influenza vaccine. Immunisation providers are trained in and follow guidelines specifically on the use of multi-dose vials.
Why should I get a COVID-19 vaccine?
- COVID-19 is a disease caused by the virus SARS-CoV-2. It can cause severe lung and generalised disease and has caused the deaths of over 2 million people worldwide since January 2020.
- Although the elderly and people with underlying medical conditions are at higher risk, anyone can get severe disease and die of COVID-19. In some people, COVID-19 may cause long-term symptoms of fatigue and breathlessness. The virus is also easily spread by people with few or no symptoms; even if you may not become unwell with COVID-19, others you may pass on the virus to can.
- By vaccinating, you are protecting yourself and others from severe COVID-19. It is also likely that once a large amount of people are vaccinated, this will decrease the spread of COVID-19 in our community.
What is COVID-19 Vaccine AstraZeneca and how does it work?
- COVID-19 Vaccine AstraZeneca is a COVID-19 vaccine developed by The University of Oxford and AstraZeneca. It contains a harmless common cold ‘carrier’ virus (an adenovirus), into which the genetic code for the SARS-CoV-2 spike protein has been inserted. The spike protein is an important part of the SARS-CoV-2 virus and helps the virus enter cells.
- After vaccination, the adenovirus carrier brings this piece of genetic code into your cells, and your cells then read it to produce copies of the spike protein. Your immune system then detects these spike proteins and learns how to recognise and fight against COVID-19.
- The adenovirus has been modified so that it cannot replicate once it is inside cells. This means that it cannot spread to other cells and cause infection. For this reason, COVID-19 Vaccine AstraZeneca is not considered a ‘live vaccine’.
How effective are COVID-19 vaccines?
- Several vaccine developers have made preliminary announcements about the efficacy of their COVID-19 vaccine in phase 3 clinical trials, and some have published early (interim) results.
- These results are very promising, and indicate that each of these vaccines is able to prevent COVID-19 disease to a statistically significant degree.
- Comirnaty (made by Pfizer) is about 95% effective at preventing people from getting sick with COVID-19, based on clinical trials and information from regulators.
- COVID-19 vaccine made by AstraZeneca/University of Oxford is about 62-70% effective at preventing people from getting sick with COVID-19, based on clinical trials and information from regulators.
- Initial clinical trial data for both the Pfizer and AstraZeneca/University of Oxford vaccine suggest very high protection (possibly close to 100%) against severe COVID-19. More data on this will come over time.
- The Novavax vaccine is about 89% effective at preventing people from getting sick with COVID-19, based on a press release from the company.
- At this stage it is unclear how long immunity from COVID-19 vaccines will last and how effectively they will decrease transmission (the spread of disease between people)
Can I have a COVID-19 vaccine if I am immunocompromised?
- Being immunocompromised means you have a weakened immune system, either from an underlying medical condition or from medical treatment that weakens your immune system.
- If you are immunocompromised, you are strongly recommended to receive either of the COVID-19 vaccines currently approved in Australia – Comirnaty (Pfizer) or COVID-19 Vaccine AstraZeneca. The ATAGI clinical guidance on COVID-19 Vaccine in Australia in 2021 provides a list of medical conditions associated with increased risk of severe COVID-19 illness.
- Both of these vaccines are considered to be safe in immunocompromised people. However, they may be less effective in immunocompromised people, because the vaccines rely on your immune system to build a response. This means that it’s important to continue other protective measures against COVID-19, even if you are vaccinated.
Can I have a COVID-19 vaccine if I have allergies?
- Almost all people with allergies can have a COVID-19 vaccine.
- The only exception is if you have had anaphylaxis (a type of severe allergic reaction) to a previous dose of a COVID-19 vaccine or to one of its ingredients.
- If you have had anaphylaxis to another substance (including foods, drugs or insect stings), or if you have been prescribed an adrenaline auto-injector (Epipen), you may be advised to stay for 30 minutes after vaccination with a COVID-19 vaccine.
If I have an allergic reaction after a COVID-19 vaccine or to one of its ingredients, can I still have the second dose?
- If you have had anaphylaxis (a type of severe allergic reaction) to a previous dose of a COVID-19, or to one of its ingredients, you should not have that vaccine again. Your healthcare provider can help to determine whether it will be safe for you to have an alternative COVID-19 vaccine.
- If you had a suspected allergic reaction which was not anaphylaxis after a COVID-19 vaccine, you may still be able to have the second dose of the vaccine, but in some cases precautions are needed such as a longer period of observation after vaccination or referral for allergy testing.
- You can find out more about the ingredients in COVID-19 vaccines in the Consumer Medicine Information, which is available on the TGA website.
Can you get COVID-19 from a COVID-19 vaccine?
- You cannot get COVID-19 from a COVID-19 vaccine.
- To get COVID-19, a live virus that can multiply in your body has to infect you. No vaccine supplied currently in the world contains live coronavirus.
- The vaccines used in Australia and elsewhere contain a genetic material that codes for the spike protein (eg, Pfizer and AstraZeneca), the spike protein itself (eg, Novavax) or an inactivated (or killed) form of the virus (in vaccines manufactured in China).
What are the likely side effects from COVID-19 vaccines?
- All vaccines can cause side effects. Usually these are mild. Clinical trials of COVID-19 vaccines have reported side effects such as pain at the injection site, fever or muscle aches starting on the day or day after vaccination.
- Comirnaty (Pfizer) is generally well tolerated and most side effects are mild and short-lived. The most common side effects include pain at the injection site, tiredness, headache, muscle pain, chills, joint pain and fever. These side effects were temporary and went away without treatment in 1-2 days.
What should I do if I have side effects after a COVID-19 vaccine?
- Your immunisation provider will tell you about the common symptoms you may experience after your COVID-19 vaccine. These may include pain, redness or swelling at the site of your injection. It may also include some general side effects such as tiredness, headache or fever. You can take paracetamol or ibuprofen to help with side effects like pain, headache or fever.
- You should seek urgent medical assistance (e.g. by calling 000) if you think you are having a severe allergic reaction, such as if you are experiencing difficulty breathing, hives, lip swelling or feeling faint.
- You should seek advice from your usual healthcare provider (e.g. GP) if you have any side effects that you are concerned about, or if your side effects have not gone away after a few days.
- You can report potential side effects after vaccination to your state or territory health authority, or directly to the Therapeutic Goods Administration (TGA). Your healthcare provider can make the report for you if you wish. This will help the TGA collect information about adverse effects that occur after COVID-19 vaccination and detect any possible unexpected safety signals.
FAQs on Flu Vaccines
What is Influenza (Flu)?
The Flu is caused by influenza viruses and affects the respiratory system, i.e. nose, throat and lungs. It is very contagious and can vary from mild to severe, and on occasion can even cause death. Complications such as bacterial pneumonia, ear infections, sinus infections and worsening of chronic medical conditions such as Asthma, Heart Failure etc can occur from the Flu virus. The Flu, during the winter season, is one of the major reasons for hospitalizations in Australia. The Flu is a very common virus, although sometimes it could cause very severe respiratory problems, and is usually prevalent during the colder months from April to October.
The Flu virus spreads when a person with the flu sneezes, coughs or talks and tiny droplets of saliva or mucus spread through the air onto other people nearby. It can also spread by touching surfaces that have the Flu virus on them and then touching your face, especially the eyes, nose and mouth.
Symptoms of the Flu include some or all of the following:
- Fever / chills
- Cough
- Sore throat
- Stuffy or runny nose
- Muscle aches
- Headache
- Fatigue
- Vomiting/diarrhoea
If you have the Flu, drinking plenty of water is highly recommended in order to keep the body from getting dehydrated. The Flu usually lasts for about one week, and antibiotics are ineffective against the flu virus.
Who is most at risk from the Flu?
- Children below 5 years
- Adults above 65 years
- Pregnant women
- Those suffering from chronic medical conditions such as HIV, Asthma, Diabetes etc
- Those with greater exposure to the virus, such as healthcare workers
How to avoid the spread of the flu virus:
- Wash hands regularly
- Cover your mouth or nose when sneezing or coughing
- Stay away from people who are sick as much as possible
- Dispose of used tissues in a hygienic way
- Do not share cups, plates, cutlery etc
- Keep surfaces clean, such as phones, keyboards etc
- Get the Flu vaccine
The Flu is an ever-evolving type of virus, and the best way to protect yourself against it is to get the annual flu vaccine, especially if you belong to one of the high-risk groups. The vaccine has proven to be very successful in reducing flu-like illnesses and the risk of complications from this virus, and even death. The vaccine causes your body to develop antibodies against several different strains of the flu virus, which help protect you from Influenza.
Side effects of the flu vaccine:
Side effects of the flu vaccine are very mild in nature and temporary, and may include:
- Swelling or redness around the point of injection
- Muscle pain
- Headache
- Mild fever (especially in children)
- Nausea
- Fatigue
On very rare occasions, the Flu vaccine has caused severe side effects, such as allergic reactions. The mild and common side effects are temporary as compared to the risk and severe health effects of the Flu virus, hence the Flu vaccine is a highly recommended preventive method for contracting the Flu.
Studies have found that if you do get a different variant of the Flu even after the Flu vaccine has been taken, the vaccine has been found to reduce the severity of the virus and symptoms.
Why does my child need a flu shot?
Flu shots, called influenza vaccines, are recommended for babies and children every year from the time they are six months old to protect them from influenza. Influenza vaccines are free for all children aged six months to under five years.
Isn’t the flu just a bad cold?
Influenza (also called ‘the flu’) can be much worse than a bad cold. Some children who have influenza get so sick they can’t go to childcare or preschool for two weeks or more. Every year in Australia, hundreds of children get so unwell from influenza they need to be treated in hospital. Most of them are babies and children under five years.
Do influenza vaccines actually work?
An influenza vaccine is the best way to protect your child from serious influenza. Influenza vaccines give better protection in some years than others. This is because the types of influenza viruses making people sick from year to year can change, and the vaccines may have to be updated. Before the influenza season, experts gather information from around the world to work out which influenza viruses are most likely to circulate. They often get it right, but sometimes it can be hard to predict. Influenza vaccines give your child good protection, even if they aren’t always perfect.
Could my child get influenza from the vaccine?
Your child can’t get influenza from an influenza vaccine. Influenza vaccines contain pieces of influenza viruses, but these can’t make your child sick like the whole virus. Some vaccines in other countries have whole, weakened influenza viruses in them, but these vaccines are not used in Australia.
It’s normal for babies and children to be a bit unsettled or even feverish for a day or two after influenza vaccination. These side-effects are a sign that your child’s immune system is responding to the vaccine. Also, the vaccine starts to protect your child after about two weeks, so if your child caught the virus before they were vaccinated (but wasn’t feeling sick yet), or in the two weeks after they were vaccinated, it might seem like the vaccine made them sick.
I’ve heard influenza vaccines can have serious side effects. Is this true?
Serious side effects are very rare. Less than two in every 100,000 children under two years have febrile convulsions (fever fits or seizures) in the days after vaccination.1 Febrile convulsions are caused by a sudden increase in body temperature. They can
be frightening, but are usually harmless. Children are much more likely to have febrile convulsions if they get sick from influenza. In one study, about four in 100 children who were treated in hospital for influenza had a febrile convulsion.2
About one child out of every million who get an influenza vaccine has a severe allergic reaction (anaphylaxis) to one of the ingredients. Any reactions usually occur before you leave the clinic, and the medical staff are trained to help children who have this reaction recover quickly. Anaphylaxis is frightening but extremely rare.
It’s safe for children with egg allergies to get influenza vaccines.3 This is because the amount of egg in influenza vaccines is tiny (usually less than one microgram of egg protein per dose, which is too small to trigger an allergic reaction). Many years ago, influenza vaccines used to contain more egg protein, but the way the vaccines are manufactured is much better now.
Does my child really need an influenza vaccine every year?
Children six months or older need to get an influenza vaccine every year. This is because the types of influenza viruses circulating often change from year to year. Also, protection from an influenza vaccine generally lasts less than a year. The best time to get an influenza vaccine is in April or May, before the influenza season, which is usually June to September. Your child can get an influenza vaccine at the same time as other vaccines.
How can we be sure the vaccine is safe?
Influenza vaccines must be assessed for safety by the Therapeutic Goods Administration (TGA) before they can be used in Australia. Although the types of influenza virus particles in vaccines may change each year, the way the vaccines are manufactured stays much the same, so they don’t need to be tested again each year. There are systems in place to detect any unexpected side-effects while a vaccine is being used. The TGA, along with other health authorities and experts, investigate any potential issues. In very rare circumstances, they may suspend use of a vaccine.
This is what happened in 2010, when one brand of influenza vaccine (Seqirus/ bioCSL Fluvax and Fluvax Junior) was found to have caused febrile convulsions in children under five years. We don’t give this specific vaccine to babies or children in Australia anymore.
FAQ on Test Results
How long does it take for my test results to come?
Results generally take 2-3 ‘working’ days to come through to your GP after you have an investigation.
Your GP then checks the results and marks them as either ‘no action’ ‘non-urgent’ or ‘urgent’.
What should I do once I have my results?
If your results are marked as ‘no action’ it means that there is no need to come back to see your GP to discuss these results. We do not alert you if your results have been marked as ‘no-action’. However, if you would like to book an appointment to discuss results with your GP, you can book one here – Hobsons Bay | Basywater
If your results are marked as “non-urgent’ this means that your GP would like to see you for an appointment to discuss your results within two weeks. You will receive an automated SMS sent via HotDoc that contains a secure link to access your results and to book an appointment to discuss with your GP.
If your results are marked as ‘urgent’ one of our team will call you directly to make an appointment with your GP, usually the same day or sometimes the next day.
FAQs on Payment Process at M3
Will I be charged before my consultation when I enter my card details?
ou will only be charged for your consultation AFTER you have had the consult and if the doctor has charged you privately rather than bulk bill.
What is the payment method at M3 Health Clinics?
We are a mixed billing medical practice and that means that if you are over the age of 15, not on a health care card or pension card, then a private payment will be charged by the GP for their services.
If you book a telehealth appointment online or via the HotDoc app you will be asked to enter your payment details before you can book an appointment.
If you book over the phone with one of our customer service team you will be sent an SMS with a secure link where you can enter your payment details. If you don’t do this within two hours of your appointment, your appointment will automatically be cancelled.
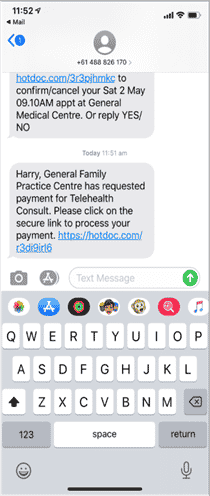
Sometimes patients see one of our GPs and payment was not collected on the day. If this happens one of our team will send you an SMS with a secure link where payment details can be entered and the payment processed.
The link will look like this.
GP Services
- 24 hour blood pressure monitoring
- 24 hour holter monitor
- 45 – 49 health assessment
- 75+ health assessments
- COVID Vaccine
- Face to face GP consultations
- Flu vaccine
- GP care plans
- Mental health care plans
- Immunisations
- Iron infusions
- Telehealth consultations
- Travel vaccinations / travel clinic
- Video Telehealth Consultations
- Yellow Fever Vaccination